We use bench-to-bedside technology to determine functional outcomes. Annabel Sorby-Adams is our own MRI expert, here teaching Hiran and the team
We use bench-to-bedside technology to determine functional outcomes. Annabel Sorby-Adams is our own MRI expert, here teaching Hiran and the team
A heart attack or a stroke occurs when a blood clot forms in an artery depriving blood flow to a region of the heart or the brain, a condition termed ischaemia. The current therapeutic standard is to reopen the artery in timely fashion, but blood flow is seldom restored before a significant amount of the heart or brain tissue has died. Because the lost tissues cannot be regenerated, the patient is left with a weakened heart often resulting in heart failure, or neurodegeneration resulting in permanent functional impairment.
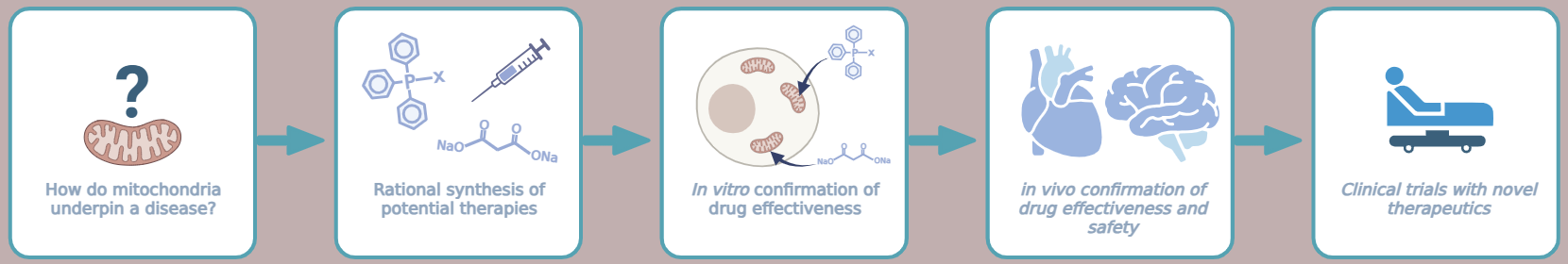
It is known that mitochondria play a pivotal role in the damage caused by ischaemia and that altering mitochondrial function can prevent ischaemic cell death. Furthermore, in addition to ischaemia itself, the restoration of blood flow (reperfusion) contributes to overall heart/brain damage, a pathological cascade termed ischaemia/reperfusion injury (IRI).
Our current research is directed at understanding the complex mechanisms underlying IRI and its consequences to brain and long-term heart function. In parallel, we apply these mechanistic insights towards identifying therapies that prevent cell death in the ischaemic heart/brain and the subsequent development of heart failure and neurological impairments.
We study these pathways using various models closely mirroring acute heart attack and stroke and the subsequent changes in addition to potentially preexisting conditions, such as diabetes, old age, or obesity. Modern imaging techniques, such as magnetic resonance imaging or positron emission tomography, help us to further understand injury and protection during IRI and to test novel drugs in such a complex setting.
We work closely with the group of Dr Michael Murphy at the MRC Mitochondrial Biology Unit who are experts in the field of specifically mitochondria-targeted therapies. Such novel therapies already showed great potential in the treatment of IRI and heart failure, and hopefully can be used soon to treat patients.
It is known that mitochondria play a pivotal role in the damage caused by ischaemia and that altering mitochondrial function can prevent ischaemic cell death. Furthermore, in addition to ischaemia itself, the restoration of blood flow (reperfusion) contributes to overall heart/brain damage, a pathological cascade termed ischaemia/reperfusion injury (IRI).
Our current research is directed at understanding the complex mechanisms underlying IRI and its consequences to brain and long-term heart function. In parallel, we apply these mechanistic insights towards identifying therapies that prevent cell death in the ischaemic heart/brain and the subsequent development of heart failure and neurological impairments.
We study these pathways using various models closely mirroring acute heart attack and stroke and the subsequent changes in addition to potentially preexisting conditions, such as diabetes, old age, or obesity. Modern imaging techniques, such as magnetic resonance imaging or positron emission tomography, help us to further understand injury and protection during IRI and to test novel drugs in such a complex setting.
We work closely with the group of Dr Michael Murphy at the MRC Mitochondrial Biology Unit who are experts in the field of specifically mitochondria-targeted therapies. Such novel therapies already showed great potential in the treatment of IRI and heart failure, and hopefully can be used soon to treat patients.